Pressure injuries (PIs) are mostly preventable, however they remain a devastating complication for patients in hospital and aged care settings, particularly where a person is immobile for a prolonged period. When a pressure injury advances to a deep wound, it can become life threatening or even fatal.
This global issue has been further exacerbated by the COVID-19 pandemic and, according to the most recent research published in The International Journal of Nursing Studies, is costing the health system billions of dollars each year. The current standard of care is a subjective assessment that can have varying accuracy levels, be time consuming, require additional staff and be disruptive to patients.
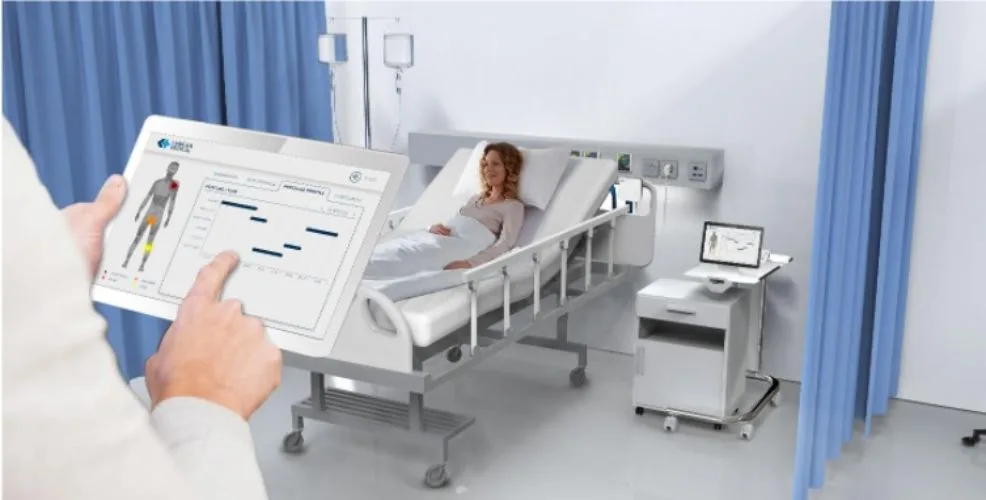
The Lenexa Medical Quality-of-Care Pressure Injury Management System is a world first medical device undergoing trials at one of the largest regional hospitals in Victoria, Bendigo Hospital. The device, which is being developed in Australia, is a fabric-based sensor which assists clinicians by providing targeted real time monitoring for patient position to enable personalised pressure injury prevention.
Bendigo Health is a 724-bed service that treats more than 49,000 inpatients on average each year, managing more than 52,000 emergency attendances in a catchment area that covers a quarter of the size of Victoria.
“Pressure injuries can develop quickly, be associated with significant pain and prolonged hospital inpatient stays with ongoing negative consequences; all of which are potentially preventable,” Bendigo Health geriatrician, Prof Marc Budge said.
“Bendigo Health’s new Hospital and residential care facilities are ideally placed to host this initial state-of-the-art technology trial. The trial aims to maximise the clinical usability of the Lenexa Medical Smart sheet system whilst optimising its integration into the clinical workflow in ICUs, hospital wards and in residential care.”
“The Lenexa solution aims to be first of its kind to help facilitate personalised and targeted pressure injury prevention, providing clinicians and carers with information vital to reduce the incidence of pressure injuries,” said Ajit Ravindran, CEO of Lenexa Medical.
“Partnering with Bendigo Health has been a great opportunity for us to demonstrate feasibility of our device with real patients in a real-world setting and helps to bring our solution closer to market. Trials will be extended to other major hospitals as well as to aged-care settings, where pressure injury prevalence is high and particularly costly”.
“It’s debilitating, your daily existence is totally consumed dealing with a pressure injury,” said Bendigo patient Norman Goetz.
“It took six months of my life coping with the painful effects. Any product that has the potential to spare anybody what I had to endure is phenomenal,” Goertz said.
“We are a global company who is invested in improving the health landscape with innovative digital solutions. We are really excited to be partnering with Lenexa Medical in a goal to improve care for patients and lessen the burden of cost for the community,” said DB Results CEO Gavin Bunshaw.
Lenexa Medical has recently been listed on the Australian Register of Therapeutic Goods (ARTG), which means it can now supply the solution to Australian Healthcare Providers.