Alcolizer Technology, a leading Australian manufacturer of diagnostic and testing solutions, today announced the results of live field trials from its highly sensitive, cost-effective rapid antigen testing device for SARS-CoV-2 (COVID-19), developed in collaboration with the University of Technology Sydney.
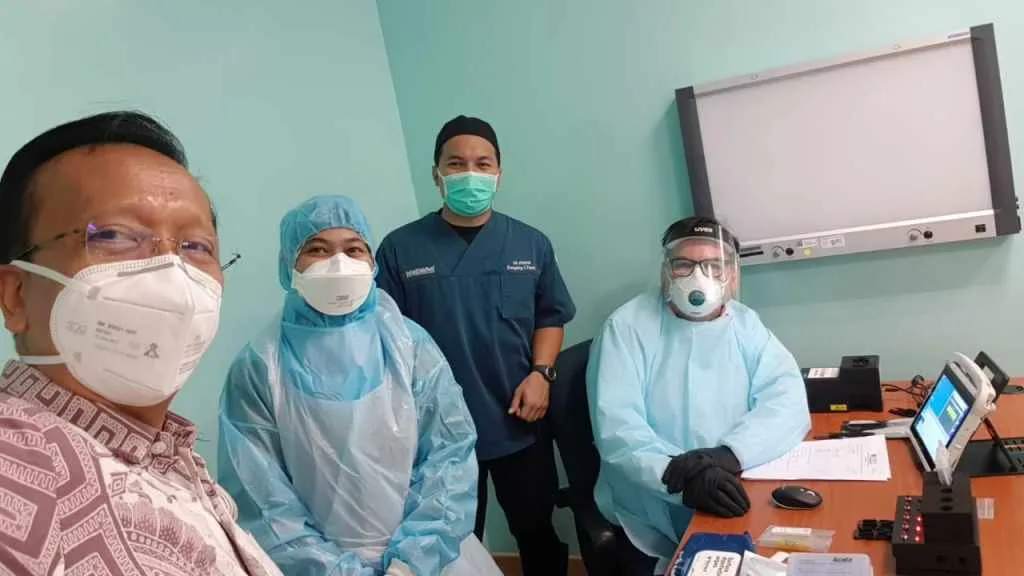
The SARS-CoV-2 Antigen Study which took place at the University Hospital, Sultan Ahmad Shah Medical Centre, Pahang Malaysia, can detect levels only previously achieved by PCR tests, and was at least 1000x more sensitive than other commercially available antigen test kits on the market.
“Live trials are a crucial component of our commercialisation strategy, and Alcolizer with support from the Advanced Manufacturing Growth Centre and the Malaysian Investment Development Authority saw the potential to conduct clinical trials in Malaysia where an Emergency Use Pathway for medical devices is in place.
What is exceptional about Virulizer TM is its non-invasive capability to detect COVID-19 in under ten minutes. Globally, antigen and PCR detection testing is a crowded and competitive space but there is still great demand to deliver a solution with the same accuracy as PCR, faster and cheaper, without the traumatic experience,” commented Roger Hunt, General Manager of Alcolizer.
Virulizer TM can detect the virus in patients who are asymptomatic or considered ‘at the point of risk’, the virus collected from a saliva sample is deactivated at the collection point to produce onsite results in under ten minutes.
“The clinical study confirmed our in-house results – that we have an exceptionally fast and sensitive platform. The platform can be modified to detect a range of infectious agents, proteins and metabolites at concentrations previously only possible using expensive clinical instruments,” commented Dr Murdo Black, Chief Scientist of Alcolizer.
“As with all viruses, we have been prepared for the evolution of mutations and continue to work closely with the Harry Perkins institute in Perth, as well as the Centenary and Kirby Institutes in Sydney to test variants as samples are made available,” commented Dr Olga Shimoni, Alcolizer’s Senior Scientist.
“Most rapid antigen tests cannot distinguish between SARS and COVID 19. The Virulizer TM antibody captures both the N and S proteins – and this gives us confidence our rapid antigen test can detect the Omicron variant, just as it did with Delta.”
Commenting on the testing method and usability experience, Dr. Kamarul Ariffin Khalid of the University Hospital Malaysia said, “It’sgreat that the virus can be deactivated at the point of risk – a gamechanger – significantly reducing transmission risk during collection and receiving results in 10 minutes instead of waiting 24 hours for the PCR.”
As infectious disease technology experts race to understand the spread of the Omicron variant in Australia’s east coast, Virulizer’s TM recent clinical success, speed of testing and high accuracy has made for a compelling investor story. Its advanced technological platform makes it ideal for contact tracing, employing cloud‐based integration of results and location tracking to monitor results.
In addition, with results pushed into the cloud Virulizer TM delivers real-time data analytics to target suburbs, pinpoint single results and monitor compliance in daily testing programs.
Having received close to $2 million in support from the Federal Government, Alcolizer is gearing up for production rollout in mid 2022. Manufacturing from its robotic facility in Balcatta it plans to make the iStrip system available to initial customers where there is an emergency use authorisation. An Australian clinical trial is set to commence with New South Wales Health in early 2022.
“Alcolizer is committed to saving lives and opening up borders, we’re confident the learnings from this study will drive a change in current thinking and process. While it won’t replace PCR testing, the sensitivity of our technology will be transformational in so many environments that require a rapid result. Imagine being able to identify an asymptomatic carrier 2-3 days before they show any sign of illness in just ten minutes. Its application to industry, health care and travel is endless,” concluded Hunt.